Your Software for
Clinical Trials
eCRF/EDC software for data collection and documentation of clinical trials and Post-Market Surveillance activities.
Study setup in 10 minutes
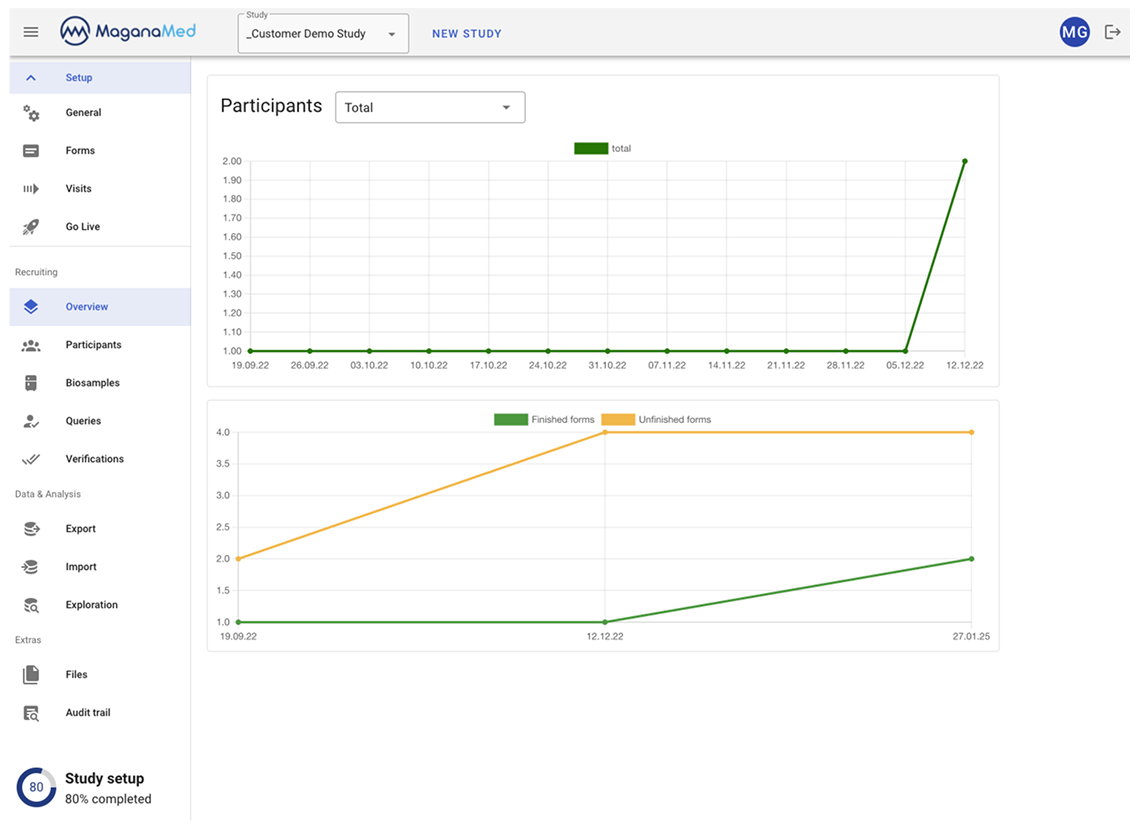
Benefits at a glance

Intuitive Operation
Our browser-based software simplifies data collection and thereby increases data quality.

Legally Secure Compliance
Full compliance with GCP, FDA 21 CFR Part 11, HIPAA, DSGVO/GDPR

Comprehensive Data Security
ISO 27001 certified data centers protect your sensitive information and reliably ensure data integrity.

Absolute predictability and transparency of costs
The price is calculated exclusively based on required functionalities/modules and duration.

Long-term Partnership
Owner-managed company with a long-term and customer-oriented corporate philosophy.

What our customers say
Experiences and feedback from direct users.
Are you interested in trying MaganaMed?